Gene expression remodelling and immune response during adaptive divergence in an African cichlid fish
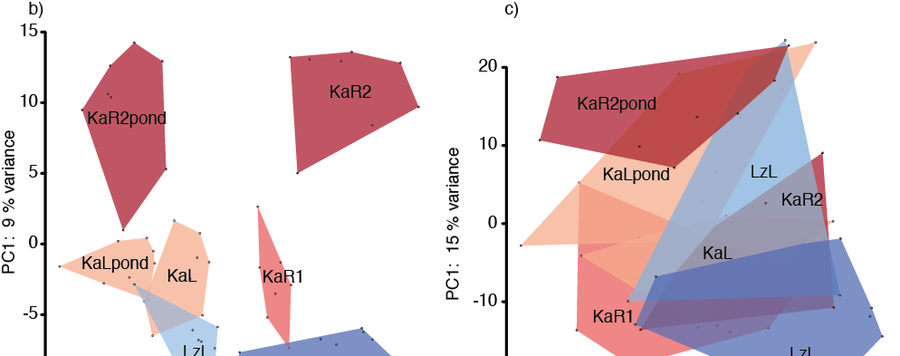
Variation in gene expression contributes to ecological speciation by facilitating population persistence in novel environments. Likewise, immune response can be a relevant factor in speciation driven by adaptation to different environments. Previous studies examining gene expression differences between recently diverged ecotypes often relied on only one pair of populations, targeted the expression of only a subset of genes, or used wild caught‐individuals. Here, we investigated the contribution of habitat‐specific parasites and symbionts and the underlying immunological capabilities of ecotype hosts to adaptive divergence in lake‐river population pairs of the cichlid fish Astatotilapia burtoni. To shed light on the role of phenotypic plasticity in adaptive divergence, we compared parasite and microbiota communities, immune response, and gene expression patterns of fish from natural habitats and a lake‐like pond setup. In all investigated population pairs, lake fish were more heavily parasitized than river fish, both in terms of parasite taxa composition and infection abundance. Innate immune response in the wild was higher in lake than in river populations and elevated in a river population exposed to lake parasites in the pond setup. Environmental differences between lake and river habitat and their distinct parasite communities shaped differential gene expression, involving genes functioning in osmoregulation and immune response. Most changes in gene expression between lake and river samples in the wild and in the pond setup were based on a plastic response. Finally, gene expression and bacterial communities of wild‐caught individuals and individuals acclimated to lake‐like pond conditions showed shifts underlying adaptive phenotypic plasticity.





