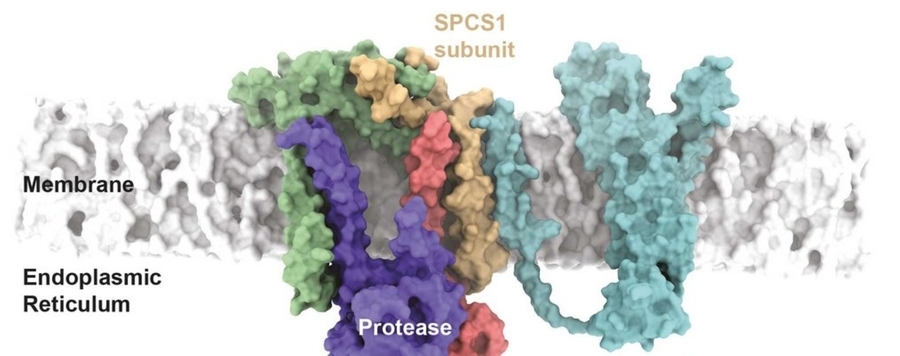
Maintenance of organelle protein homeostasis by membrane proteases and pseudoproteases
Protein homeostasis mechanisms are fundamentally important to match cellular needs and to counteract stress conditions. A fundamental problem is to understand how defective proteins are recognized and extracted from cellular organelles to be degraded in the cytoplasm. The ER-associated degradation (ERAD) pathway is the best-understood organellar protein quality control system. A number of studies have identified key players of the ERAD machinery, comprising polytopic E3 ubiquitin ligases and derlins, which jointly facilitate recognition, ubiquitination, and retrotranslocation of faulty proteins to prompt their degradation by the cytoplasmic proteasome. As a variation of the theme, we found that the ER-resident proteases including rhomboids, SPP and the signal peptidase complex drive degradation of selected ERAD substrates by introducing an irreversible cut that serves as a signal for subsequent retrotranslocation and degradation. Our current models of how proteolysis, in the context of the ER membrane, complements the ubiquitin code of the ERAD pathway will be discussed.





